
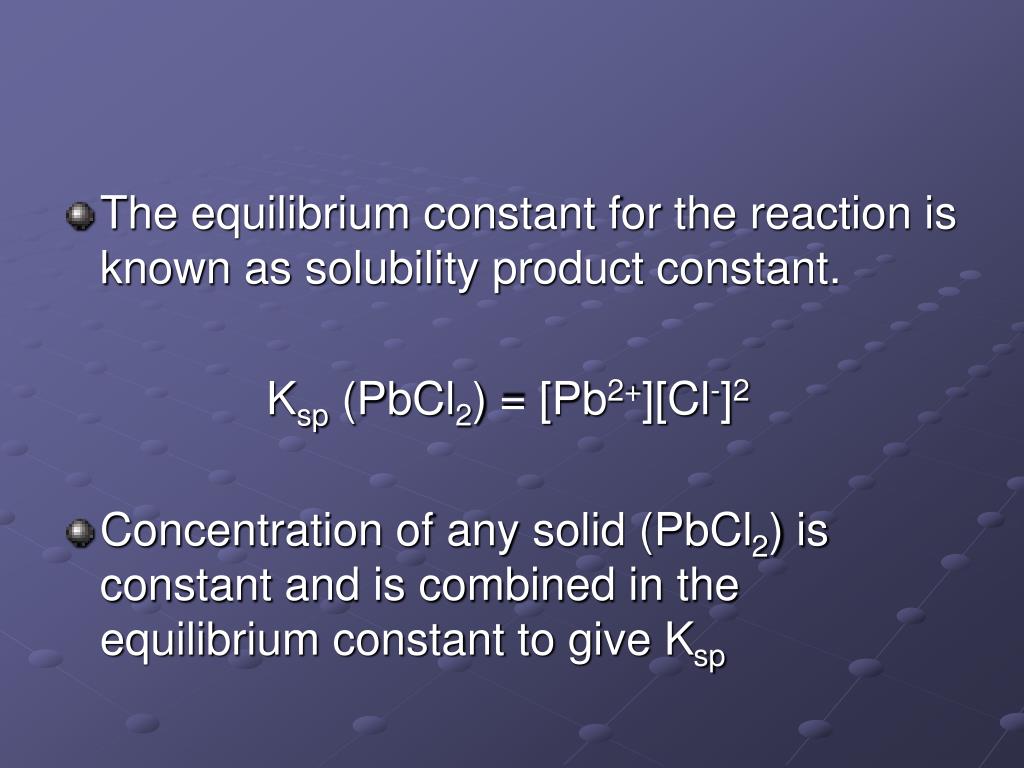
There are many other factors that can affect solubility, but these rules are a good first step to determine the outcome of aqueous solution reactions. The solubility rules are a useful guideline to predict whether a compound will dissolve or form a precipitate. The finished reaction is:Ģ KCl(aq) + Pb(NO 3) 2(aq) → 2 KNO 3(aq) + PbCl 2(s) This means PbCl 2 is insoluble and form a precipitate. Chlorides are soluble in water with the exception of silver, lead and mercury. KNO 3 will remain in solution since all nitrates are soluble in water. A listing of solubility product constants for several sparingly soluble compounds is provided in Appendix J. Now, given that the molar solubility is 2.2 x 10-3 M, you can plug this into the equation for both F - and Ca 2+. Remember: The F - must be raised to the second power due to the coefficient in the balanced equation. KCl(aq) + Pb(NO 3) 2(aq) → KNO 3(?) + PbCl 2(?)Ģ KCl(aq) + Pb(NO 3) 2(aq) → 2 KNO 3(?) + PbCl 2(?) AgCl(s) Ag+(aq) +Cl(aq) Ksp Ag+(aq)Cl(aq) Recall that only gases and solutes are represented in equilibrium constant expressions, so the Ksp does not include a term for the undissolved AgCl. Given molar solubility for the ions in question and the balanced equation, you can find the K sp. The products should rearrange the ions to: What would be the expected products and will a precipitate form? The resulting balanced reaction would be:Ģ AgNO 3(aq) + MgBr 2 → 2 AgBr(s) + Mg(NO 3) 2(aq) The other compound Mg(NO 3) 2 will remain in solution because all nitrates, (NO 3) -, are soluble in water. Are the products soluble in water?Īccording to the solubility rules, all silver salts are insoluble in water with the exception of silver nitrate, silver acetate and silver sulfate. The state of the products needs to be determined. The balanced reaction would be:Ģ AgNO 3(aq) + MgBr 2 → 2 AgBr(?) + Mg(NO 3) 2(?) Since Ag + is now in solution with Cl-the two will combine to form AgCl, and the AgCl will precipitate from solution. The resulting solution contains Na +, Ag +, Cl-, and NO 3-, but AgCl is not soluble in water. Dry your product thoroughly and re-weight it to get the true percent yield. A solution of silver nitrate is combined with a solution of sodium chloride. A note about the values obtained a value above 100 100 100 is possible but is due to solvent being present in the sample as well as your product. For example, a silver nitrate solution (AgNO 3) is mixed with a solution of magnesium bromide (MgBr 2). Or you could use our percent yield calculator to calculate it easily and quickly. The question remains, will AD or CB remain in solution or form a solid precipitate?Ī precipitate will form if the resulting compound is insoluble in water. This reaction is generally a double replacement reaction in the form: In the previous two examples, we have seen that Mg(OH) 2 or AgCl precipitate when Q is greater than K sp. When two aqueous solutions are mixed, the ions interact to form products. These solutions are represented in chemical equations in the form: AB(aq) where A is the cation and B is the anion.
AGCL PRECIPITATE CALCULATOR HOW TO
This guide will show how to use the solubility rules for inorganic compounds to predict whether or not the product will remain in solution or form a precipitate.Īqueous solutions of ionic compounds are comprised of the ions making up the compound dissociated in water. \) ions will remain in the solution.When two aqueous solutions of ionic compounds are mixed together, the resulting reaction may produce a solid precipitate.


 0 kommentar(er)
0 kommentar(er)
